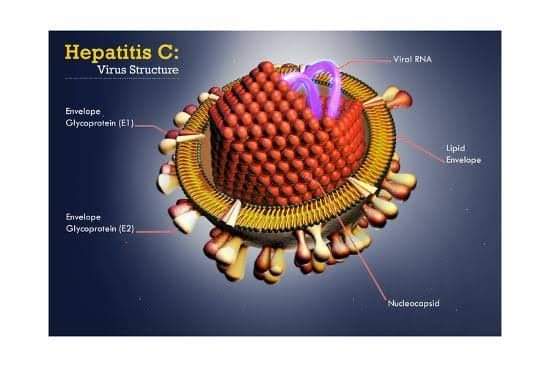
Hepatitis C Virus:
Hepatitis C virus (HCV) one of the most common bloodborne pathogen. In 2016, the World Health Organization developed an international strategy and action plans to neutralize the effect of HCV by 2030. Eradication of HCV is a national goal. Increased efforts to extend access to treatment to populations that traditionally are difficult to treat, such as persons who inject drugs, are critical to achieving eradication.
👉What factors increase risk for HCV infection?
Most persons with HCV were infected via a percutaneous exposure to infected blood.
The 2 primary methods of bloodborne transmission are:
➡Injection drug use
➡Nosocomial exposure
Sharing of injection equipment contaminated with infected blood, such as needles and syringes, among persons who inject drugs frequently results in exposure and transmission.
HCV may be transmitted via sexual activity, given that HCV RNA is occasionally detected in the semen of viremic patients. HCV seems to be transmitted at a higher rate in men who have sex with men (MSM), particularly those who practice unprotected anal intercourse or have HIV. HCV may be transmitted from mother to child at a rate of approximately 4%–5%. Breastfeeding is not associated with transmission and is considered safe.
👉How can risk be reduced in the health care setting?
To minimize the risk for patient-to-patient transmission, blood banks should avoid paid donors and should routinely screen donated products. Health care workers and volunteers should follow infection control practices that prevent reuse of contaminated equipment.
🔴Screening:
Screening for HCV infection is recommended because both the acute and chronic phases are mostly asymptomatic. There is a long latent period usually decades before liver disease manifests, during which treatment can almost entirely prevent complications. Initial HCV serologic testing is relatively inexpensive.
Populations that should be screened:
Injection drug use (current or ever, including those who have injected once).
➡Persons who have ever received long-term hemodialysis.
➡Health care, emergency medical, and public safety workers after sticks with needles or sharps or mucosal exposure to HCV-infected blood.
➡Children born to HCV-infected women.
➡Persons with HIV infection
➡Persons with unexplained chronic liver disease and chronic hepatitis, including elevated ALT levels.
➡Solid organ donors (deceased and living).
🔴Diagnosis and Evaluation:
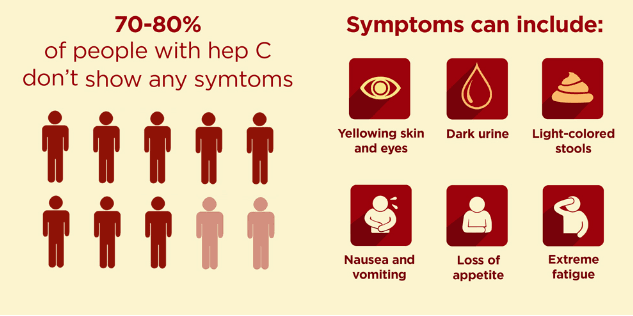
The incubation period after exposure to HCV is typically 2–12 weeks, during which time HCV RNA is detectable and transmissible but liver enzyme levels, such as alanine aminotransferase (ALT), remain normal. After incubation, mild acute hepatitis occurs, during which most persons remain asymptomatic. 10%–15% develop flu-like symptoms, myalgias, jaundice, or dark urine.
Given this largely asymptomatic natural history, the vast majority of persons with acute infection do not present for medical care. Anti-HCV antibodies (HCVAbs) may still remain undetectable in the early acute phase of infection; therefore, detection at this phase requires HCV RNA testing.
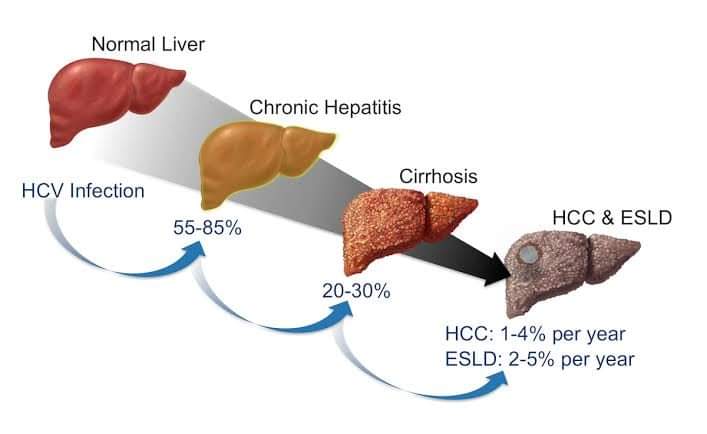
Most patients without treatment in the acute phase will progress to chronic infection. During chronic infection, levels of ALT are usually persistently or intermittently elevated but can be normal in up to 20% of infected persons.
Chronic infection is usually asymptomatic but may result in extrahepatic manifestations.
Extrahepatic Manifestations of HCV Infection:
➡Cryoglobulinemic vasculitis
➡Membranoproliferative glomerulonephritis
➡Membranous nephropathy
➡Monoclonal gammopathy
➡Non-Hodgkin lymphoma
➡Arthralgias/arthritis
➡Raynaud phenomenon
➡Fatigue
➡Sicca syndrome
➡Lichen planus
➡Porphyria cutanea tarda
➡Diabetes mellitus/insulin resistance
➡Hypothyroidism/hyperthyroidism
The major consequence of chronic infection is liver fibrosis that can lead to cirrhosis over several decades. On average, 15%–20% of persons with chronic HCV infection develop cirrhosis over a 20-year period.
Once cirrhosis develops, the patient may have significant morbidity and mortality due to
portal hypertension, hepatic encephalopathy and hepatocellular carcinoma.
What are symptoms and signs to look for on physical examination?
Symptoms of chronic liver disease can include:
➡Fatigue
➡Pruritus
➡Weakness
➡Hair loss
➡Anorexia
Signs of cirrhosis include:
➡Scleral icterus
➡Spider nevi on the chest or back
➡Palmar erythema
➡Terry nails
➡Temporal wasting
➡Thenar wasting
➡Reduced body hair
➡Gynecomastia
➡Peripheral edema
➡Dilated abdominal veins
➡Hepatomegaly
➡Splenomegaly
➡Ascites
👉What diagnostic tests are available?
Patients should be screened with an anti-HCV (HCVAb) test using either a blood-based or point-of-care enzyme-linked immunoassay. These tests have high specificity and sensitivity.
Tests for HCVAbs are less expensive than nucleic acid testing (NAT), but they do not differentiate current from past infection and can show false-positive results. NAT is the preferred first test in the acute setting and for immunocompromised persons who may exhibit delayed HCVAb development. Once a positive HCVAb test result is identified, measurement of HCV RNA, usually by polymerase chain reaction, is used to confirm current infection.
What are common laboratory test abnormalities that might suggest advanced fibrosis or cirrhosis?
Fibrosis-4 (FIB-4) is now recommended as a component of the pretreatment assessment by American Association for the Study of Liver Diseases (AASLD) guidelines.
A FIB-4 score greater than 3.25 is highly specific for advanced fibrosis, including cirrhosis. Thus, risk stratification for advanced fibrosis can easily be initiated during the first clinical encounter for most patients newly diagnosed with HCV infection.
FIB-4 Calculation:
FIB-4 = [age (years) × AST level (U/L)] / [platelet count (×109 cells/L) × √ALT level (U/L)]
🔹FIB-4 > 3.25: 97% specific for cirrhosis
🔹FIB-4 < 1.45: 90% specific for absence of advanced fibrosis
🔹1.45 ≤ FIB-4 ≤ 3.25: Indeterminate; additional testing needed.
👉How is the degree of fibrosis determined?
Determining the fibrosis stage helps predict morbidity and mortality risk. There are several fibrosis scoring systems, but the most commonly used is METAVIR (Meta-analysis of Histological Data in Viral Hepatitis) which stages patients from F0 (no fibrosis) to F4 (cirrhosis).
Grading:
🔸Score F0: No fibrosis.
🔸Score F1: Minimal scarring.
🔸Score F2: Positive scarring and extension beyond area containing blood vessels.
🔸Score F3: Bridging fibrosis connecting other areas of fibrosis.
🔸Score F4: Cirrhosis or advanced liver scarring.
HBV/HCV co-infection:
Hepatitis C typically suppresses hepatitis B replication in patients who are co-infected. HCV suppression can thus lead to HBV reactivation (HBVr). Patients who are HBsAg-positive should be treated with an HBV-directed DAA (Direct-Acting Antiviral) during and after HCV treatment. These patients should be routinely monitored for HBVr for at least 24 weeks after completion of HCV treatment and if liver test results become abnormal thereafter.
Reference: American College of Physicians (ACP) journals.
Platform Academic Division/ Dipta Dash Shuva
Army Medical College Chattogram
2016-17